Interesting facts from the world of organic chemistry
Derivatives - carbonyl compounds I:
Estrone
Together with estradiol and estriol, estrone belongs to the family of reproductive hormones. These substances have been discussed previously. Estrone is also called oestrone, also abbreviated E1. We can also come across names such as aquacrine, erystogen or menagen. The meaning of the abbreviation E1 depends on the structure of estrone – in its molecule, estrone contains only one hydroxyl group.
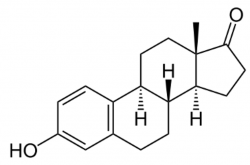
Picture above: Chemical structure of estrone.
Estrone, in its pure form a white crystalline powder with a melting point of around 255 °C, is the least represented hormone in the female body of the three named. As one of the estrogens, it belongs to a group of substances that, strangely enough, are also known as carcinogens. The effects depend on the size of the received dose. In women with a normal menstrual cycle, a day will release from (70 to 500) μg of estradiol, depending on the phase of the cycle. This estradiol is mainly converted to estrone and a small amount of estriol.
The synthesis of estrone is possible, for example, from androstenedione (see figure below), which in turn comes from cholesterol.
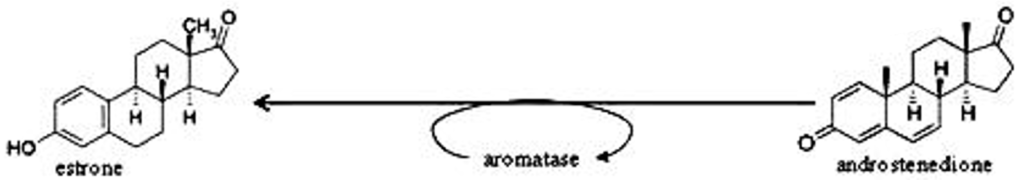
Picture above: Last step in the synthesis of androstendion*.
*Androstendion is a steroid hormone.
Sources:
[1] V. Rochira, B. Madeo, C. Diazzi, L. Zirilli, D. Santi a C. Carani, „Estrogens and Male Reproduction,“ Endotext.com, 6 2 2013. [Online]. Available: http://www.endotext.org/male/male17/male17.htm. [Přístup získán 7 6 2013].
[2] W. F. Boron a E. L. Boulpaep, v Medical Physiology: A Cellular And Molecular Approach, Saunders, 2003, p. 1300.
[3] E. K. Achyuthan, P. D. Adams, S. Datta, B. A. Simmons a A. K. Singh, „Molecules,“ 2010. [Online]. Available: http://www.mdpi.com/1420-3049/15/3/1645. [Přístup získán 7 6 2013].
[4] J. Ralph a Y. Zhang, „Agricultural Research Service,“ 1998. [Online]. Available: http://www.dfrc.ars.usda.gov/DFRCWebPDFs/1998-Ralph-Tet-54-1349.pdf. [Přístup získán 7 6 2013].
[5] M. Bolognini, F. Cavani, L. D. Pozzo, L. Maselli, F. Zaccarelli, B. Bonelli, M. Armandi a E. Garrone, „ScienceDirect,“ 21 5 2004. [Online]. Available: http://www.sciencedirect.com/science/article/pii/S0926860X04004703. [Přístup získán 7 6 2013].
[6] J. Moravcová, v Biologicky aktivní přírodní látky, Praha, VŠCHT Praha, 2006, pp. 33-34.
[7] OSHA, „United States Department of Labor,“ 30 3 2007. [Online]. Available: http://www.osha.gov/dts/chemicalsampling/data/CH_238925.html. [Přístup získán 7 6 2013].
[8] „National Toxicology Program,“ 2011. [Online]. Available: http://ntp.niehs.nih.gov/ntp/roc/twelfth/profiles/EstrogensSteroidal.pdf. [Přístup získán 7 6 2013].
